Collect data in a streamlined, compliant and efficient way.
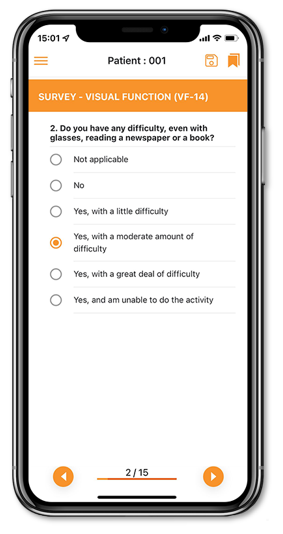
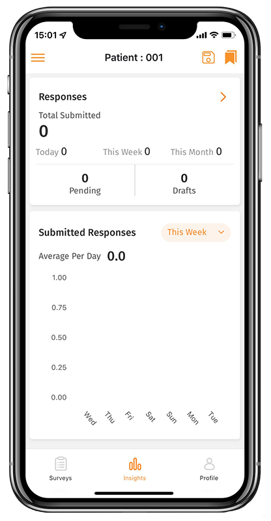
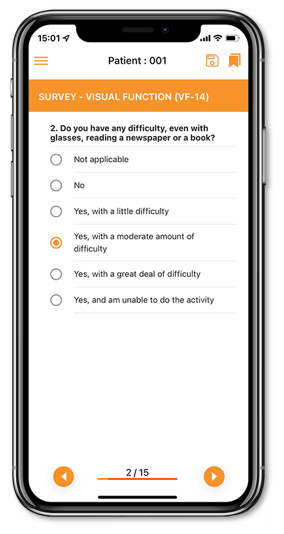
Our electronic Clinical Outcome Assessment (eCOA) solution optimizes real-time, data collection directly from patients by offering a simple and intuitive interface providing advanced patient assessments to enhance the patient experience and improve data quality.
eCOA by Meditrial brings data science services and technology together.
Customers can benefit from an end-to-end comprehensive eCOA package or create value by selecting from multiple options in order to meet case by case requirements.
eCOA has been created to collect any patient/clinician/observer reported outcome data in a streamlined, compliant and efficient way.
KEY FEATURES
- Customized eCOA App to deliver a step-by-step online guidance for the operator
- Online training recording for the investigator
- Automated score calculation, e.g. Detection Threshold (DT)
- Multilanguage functionality
- Complete audit trail ensuring GCP and FDA compliant data capture
- One-click data exports through the central interface
Accelerate your path to decentralized clinical trials
Meditrial eCOA is revolutionizing the way sponsors, CROs, and sites collect electronic data from patients, physicians and caregivers.
eCOA provides a flexible, intuitive model for capturing patient data that is designed to make it easier for patients to engage in clinical trials. Built as part of the Catchtrial© platform, eCOA improves your study experience with flexible deployment options, a groundbreaking global instrument library, and dedicated services and support.
Available as an iOS or Android app or web-based solution.